Selected
- Wang S, Zhang P, Sun Y, Fang Y, Wang P, Shao M, Zhang N, Shi S, Chen X, Gao H, Cheng J, Gao B, Liu T, Qian Q, Song C. Engineering of BZ transposase and transposon donor vector for enhanced efficiency and safety in gene delivery applications. Nucleic Acids Res. 2025 Sep 23;53(18):gkaf935. https://academic.oup.com/nar/article/53/18/gkaf935/8262245.
- Diaby, M., Wu, H., Gao, B., Shi, S., Wang, B., Wang, S., Wang, Y., Wu, Z., Chen, C., Wang, X., & Song, C*. (2024). A Naturally Active Spy Transposon Discovered from the Insect Genome of Colletes gigas as a Promising Novel Gene Transfer Tool. Advanced science, e2400969. https://doi.org/10.1002/advs.202400969
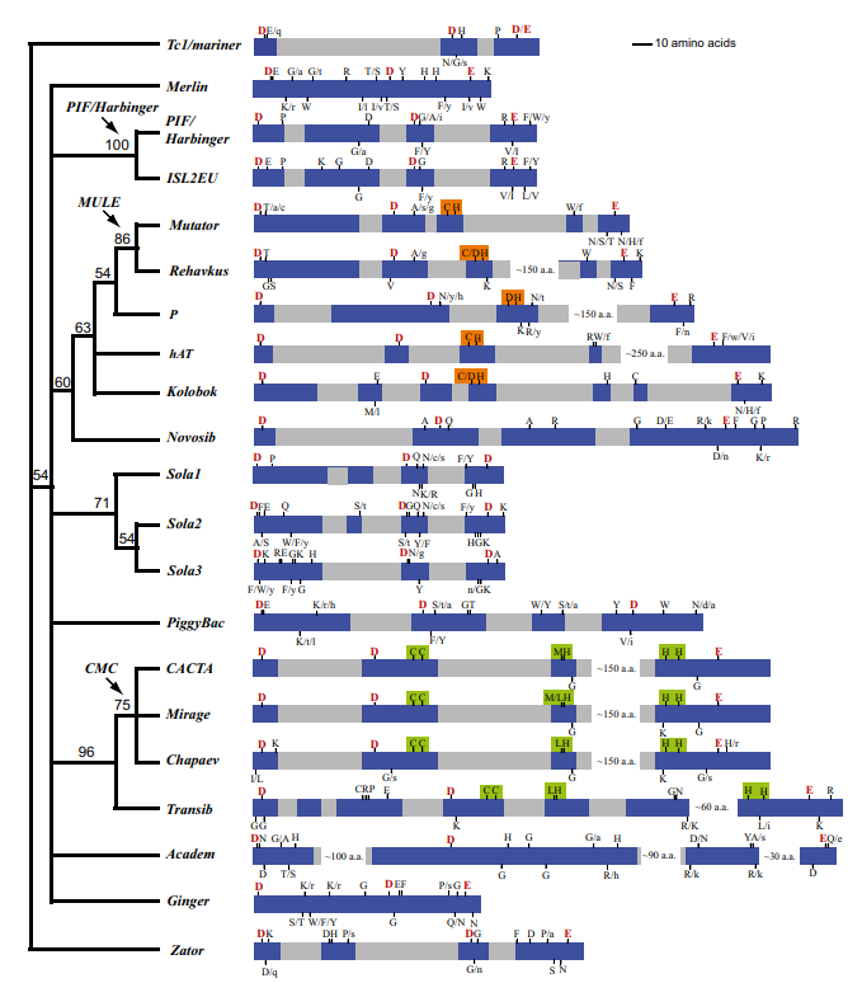
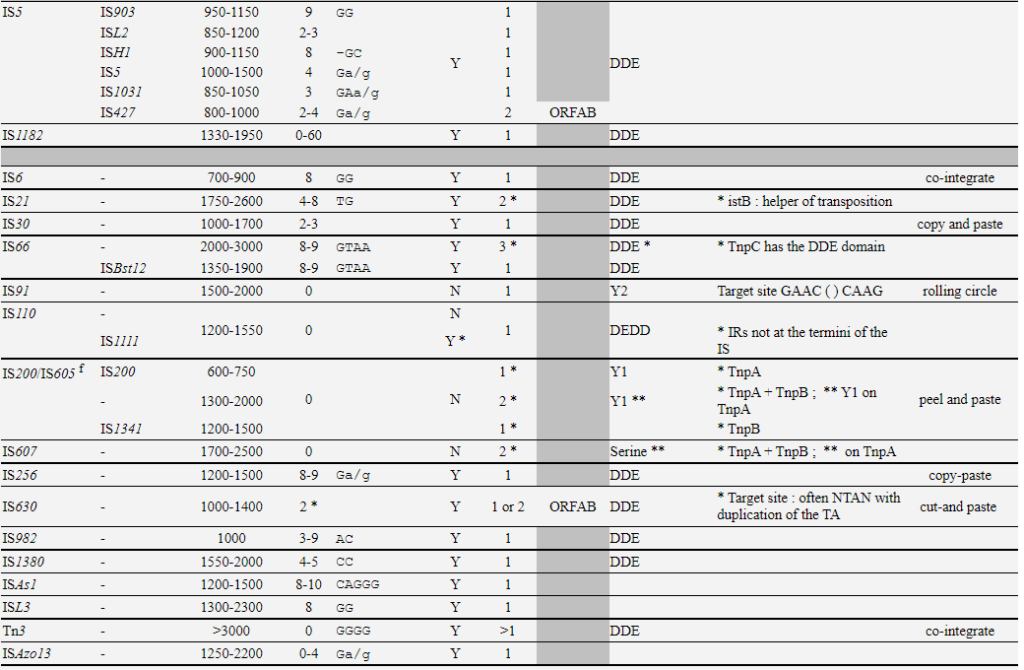
- SONG, C. and IVICS, Z. (2024). Transposable Elements as Tools. In Transposable Elements and Genome Evolution (eds A. Hua-Van and P. Capy). https://doi.org/10.1002/9781394312467.ch10
- Saisai Wang, Bo Gao, Csaba Miskey, Zhongxia Guan, Yatong Sang, Cai Chen, Xiaoyan Wang, Zoltán Ivics, Chengyi Song*, Passer, a highly active transposon from a fish genome, as a potential new robust genetic manipulation tool, Nucleic Acids Research, 2023;, gkad005, https://doi.org/10.1093/nar/gkad005
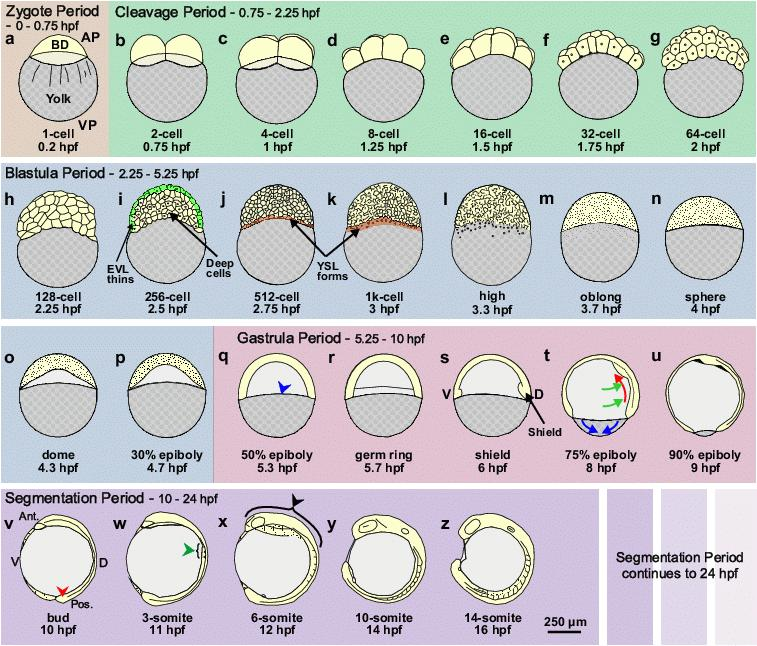
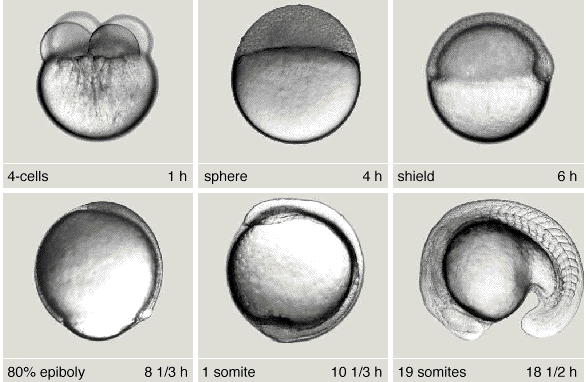
- Dan Shen, Chengyi Song, Csaba Miskey, Shuheng Chan, Zhongxia Guan, Yatong Sang, Yali Wang, Cai Chen, Xiaoyan Wang, Ferenc Müller, Zoltán Ivics, Bo Gao* , A native, highly active Tc1/mariner transposon from zebrafish (ZB) offers an efficient genetic manipulation tool for vertebrates, Nucleic Acids Research, 2021 Feb 26;49(4):2126-2140. https://doi.org/10.1093/nar/gkab045.
2025
- Wang S, Zhang P, Sun Y, Fang Y, Wang P, Shao M, Zhang N, Shi S, Chen X, Gao H, Cheng J, Gao B, Liu T, Qian Q, Song C. Engineering of BZ transposase and transposon donor vector for enhanced efficiency and safety in gene delivery applications. Nucleic Acids Res. 2025 Sep 23;53(18):gkaf935. doi: 10.1093/nar/gkaf935.
- Zishuai Wang, Zixin Li, Tao Huang, Jianhai Chen, Pan Xu, Ruimin Qiao, Hongwei Yin, Chengyi Song, Dongjie Zhang, Di Liu, Shuhong Zhao, Martien A.M. Groenen, Ole Madsen, Yanlin Zhang, Lijing Bai, Kui Li, Genomic insights into the demographic history and local adaptation of wild boars across Eurasia, Cell Genomics, 2025, https://doi.org/10.1016/j.xgen.2025.100954.
- Ahmed A. Saleh, Naisu Yang, Amr M.A. Rashad, Nada N.A.M. Hassanine, Ali Shoaib Moawad, Bo Gao, Chengyi Song, Evolution of endogenous retroviruses (ERVs) in the Bovinae subfamily, Gene, Volume 965, 2025, 149663, https://doi.org/10.1016/j.gene.2025.149663.
- Zong, W., Chen, L., Zhang, D., Zhang, Y., Wang, J., Hou, X., Chai, J., An, Y., Tian, M., He, X., Song, C., He, J., Liu, X., Wang, L., D’Alessandro, E., Wang, L., Yin, Y., Li, M., Liu, D., Wang, J., … Zhang, L. (2025). Two telomere-to-telomere pig genome assemblies and pan-genome analyses provide insights into genomic structural landscape and genetic adaptations. iMeta, 4(2), e70013. https://doi.org/10.1002/imt2.70013
- Cai Chen, Mengli Wang, Yao Zheng, Ziyan Liu, Phiri Azele, Ahmed A. Saleh, Xiaoyan Wang & Chengyi Song. A 280 bp SINE insertion within the pig PLA2G16 could potentially modify gene expression through integration with its transcript. J Appl Genetics (2025). https://doi.org/10.1007/s13353-024-00933-5
- Saleh, A. A., Moawad, A. S., Yang, N., Zheng, Y., Chen, C., Wang, X., Gao, B., & Song, C. (2025). Association of a 7.9 kb Endogenous Retrovirus Insertion in Intron 1 of CD36 with Obesity and Fat Measurements in Sheep. Mobile DNA, 16(1), 12. https://doi.org/10.1186/s13100-025-00349-w
- Wang Xiaoyan , Zhou Chenyu , Zheng Yao , Yu Miao , He Jia , Chen Cai , Qiao Suwei , Moawad Ali Shoaib , Tian Guoxing , Li Bixia , Song Chengyi. Population structure and genetic diversity of Mi pigs based on SINE-RIPs, Frontiers in Veterinary Science, Volume 12 – 2025, https://doi.org/10.3389/fvets.2025.1500115
- Wang Q, Shi S, Wang B, Chen X, Yang N, Gao B, Song C. Evolution Landscape of PiggyBac (PB) Transposon in Beetles (Coleoptera). Genes (Basel). 2025 Dec 18;16(12):1521. doi: 10.3390/genes16121521. PMID: 41465194; PMCID: PMC12732532.
- Zhou C, Qiao S, Zheng Y, Yu M, Chen H, Chen C, Moawad AS, Gao B, Song C, Wang X. Impact of the 294 bp SINE Insertion in 5’UTR of the GLYATL3 Gene on Gene Expression and Phenotypic Variation. Animals (Basel). 2025 May 9;15(10):1375. doi: 10.3390/ani15101375. PMID: 40427253; PMCID: PMC12108459.
- Addy GA, Wang Q, Chen H, Abdu W, Diaby M, Chen X, Asare E, Gao B, Song C. Evolution of Mutator transposons in the genomes of worms. Gene. 2026 Jan 10;975:149855. doi: 10.1016/j.gene.2025.149855. Epub 2025 Oct 30. PMID: 41173222.
- Guan Z, Wang B, Gao B, Shi S, Wang S, Wu H, Diaby M, Chen C, Wang X, Song C. Multiple Buster transposons from hAT superfamily displaying transposition activities in mammal cells. Synth Syst Biotechnol. 2025 Nov 4;11:397-406. doi: 10.1016/j.synbio.2025.10.006. PMID: 41280276; PMCID: PMC12637253.
- Saleh A, Yang N, Wang F, Rashad A, Moawad A, Chen C, Wang X, Gao B, Song C. Mining and identification of solitary long terminal repeat (Solo-LTR) presence polymorphisms in the sheep genomes. Genome. 2025 Jan 1;68:1-16. doi: 10.1139/gen-2025-0012. PMID: 41213126.
2024
- SONG, C. and IVICS, Z. (2024). Transposable Elements as Tools. In Transposable Elements and Genome Evolution (eds A. Hua-Van and P. Capy). https://doi.org/10.1002/9781394312467.ch10
- Diaby, M., Wu, H., Gao, B., Shi, S., Wang, B., Wang, S., Wang, Y., Wu, Z., Chen, C., Wang, X., & Song, C*. (2024). A Naturally Active Spy Transposon Discovered from the Insect Genome of Colletes gigas as a Promising Novel Gene Transfer Tool. Advanced science, e2400969. https://doi.org/10.1002/advs.202400969
- Wencheng Zong, Runze Zhao, Xiaoyan Wang, Chenyu Zhou, Jinbu Wang, Cai Chen, Naiqi Niu, Yao Zheng, Li Chen, Xin Liu, Xinhua Hou, Fuping Zhao, Ligang Wang, Lixian Wang*, Chengyi Song*, Longchao Zhang*, Population genetic analysis based on the polymorphisms mediated by transposons in the genomes of pig, DNA Research, 2024;, dsae008, https://doi.org/10.1093/dnares/dsae008
- Moawad, Ali Shoaib, Fengxu Wang, Yao Zheng, Cai Chen, Ahmed A. Saleh, Jian Hou, and Chengyi Song*. 2024. Evolution of Endogenous Retroviruses in the Subfamily of Caprinae. Viruses 16, no. 3: 398. https://doi.org/10.3390/v16030398
- Guo, Mengke, George A. Addy, Naisu Yang, Emmanuel Asare, Han Wu, Ahmed A. Saleh, Shasha Shi, Bo Gao, and Chengyi Song*. 2024. PiggyBac Transposon Mining in the Small Genomes of Animals. Biology 13, no. 1: 24. https://doi.org/10.3390/biology13010024
- Saisai Wang, Zhongxia Guan, Mohamed Diaby, Emmanuel Asare, Numan Ullah, Wenzhu Jia, Bo Gao, Duonan Yu, Chengyi Song*, Evolution of Skipper (SK), a family of DD34E/Tc1 transposons, in animals, Biological Journal of the Linnean Society, 2023;, blad141, https://doi.org/10.1093/biolinnean/blad141.
- Du, Zhanyu, Cai Chen, Yao Zheng, Xiaoyan Wang, and Chengyi Song. 2024. Retroviral Insertion Polymorphism (RIP) of Porcine Endogenous Retroviruses (PERVs) in Pig Genomes. Animals 14, no. 4: 621. https://doi.org/10.3390/ani14040621
- 5) Wang, Bingqing, Ahmed A. Saleh, Naisu Yang, Emmanuel Asare, Hong Chen, Quan Wang, Cai Chen, Chengyi Song, and Bo Gao*. 2024. High Diversity of Long Terminal Repeat Retrotransposons in Compact Vertebrate Genomes: Insights from Genomes of Tetraodontiformes. Animals 14, no. 10: 1425. https://doi.org/10.3390/ani14101425
- Book: Livestock Genome Editing Tools, 3. Associated technologies for genome editing, 2024, ISBN: 978-0-12-819099-9 https://doi.org/10.1016/C2018-0-04416-3
- Moawad, Ali Shoaib, Fengxu Wang, Yao Zheng, Cai Chen, Ahmed A. Saleh, Jian Hou, and Chengyi Song*. 2024. Evolution of Endogenous Retroviruses in the Subfamily of Caprinae. Viruses 16, no. 3: 398. https://doi.org/10.3390/v16030398
- Chen, Cai, Zhanyu Du, Yao Zheng, Hong Chen, Ahmed A. Saleh, Naisu Yang, Mengli Wang, Phiri Azele, Xiaoyan Wang, and Chengyi Song*. 2024. Investigation of Polymorphisms Induced by the Solo Long Terminal Repeats (Solo-LTRs) in Porcine Endogenous Retroviruses (ERVs). Viruses 16, no. 11: 1801. https://doi.org/10.3390/v16111801
2023
- Saisai Wang, Bo Gao, Csaba Miskey, Zhongxia Guan, Yatong Sang, Cai Chen, Xiaoyan Wang, Zoltán Ivics, Chengyi Song*, Passer, a highly active transposon from a fish genome, as a potential new robust genetic manipulation tool, Nucleic Acids Research, 2023;, gkad005, https://doi.org/10.1093/nar/gkad005
- Hao Gu, Zhan-yu Du, Eduard Murani, Enrico D’Alessandro, Cai Chen, Xiao-yan Wang, Jiu-de Mao, Klaus Wimmers, Chengyi Song*, A 314-bp SINE insertion in the ZNF2 promoter region may act as a repressor related to regulation of fat deposition in pigs, Journal of Integrative Agriculture, Volume 22, Issue 2, 2023, Pages 526-536, ISSN 2095-3119, https://doi.org/10.1016/j.jia.2022.08.128.
- Li, Xueyuan, Zhongxia Guan, Feng Wang, Yali Wang, Emmanuel Asare, Shasha Shi, Zheguang Lin, Ting Ji, Bo Gao, and Chengyi Song*. 2023. Evolution of piggyBac Transposons in Apoidea. Insects 14, no. 4: 402. https://doi.org/10.3390/insects14040402
- Wang, Yali, Mengke Guo, Naisu Yang, Zhongxia Guan, Han Wu, Numan Ullah, Emmanuel Asare, Shasha Shi, Bo Gao, and Chengyi Song*. 2023. Phylogenetic Relationships among TnpB-Containing Mobile Elements in Six Bacterial Species. Genes 14, no. 2: 523. https://doi.org/10.3390/genes14020523
- Zheng, Yao, Cai Chen, Mengli Wang, Ali Shoaib Moawad, Xiaoyan Wang, and Chengyi Song*. 2023. SINE Insertion in the Pig Carbonic Anhydrase 5B (CA5B) Gene Is Associated with Changes in Gene Expression and Phenotypic Variation. Animals 13, no. 12: 1942. https://doi.org/10.3390/ani13121942
- Xiang, Kuilin, Mikhail Puzakov, Shasha Shi, Mohamed Diaby, Numan Ullah, Bo Gao, and Chengyi Song*. 2023. Mosquito (MS), a DD37E Family of Tc1/Mariner, Displaying a Distinct Evolution Profile from DD37E/TRT and DD37E/L18. Genes 14, no. 7: 1379. https://doi.org/10.3390/genes14071379
- Shasha Shi, Mikhail V. Puzakov, Ludmila V. Puzakova, Yulia N. Ulupova, Kuilin Xiang, Binqing Wang, Bo Gao, Chengyi Song*, Hiker, a new family of DNA transposons encoding transposases with DD35E motifs, displays a distinct phylogenetic relationship with most known DNA transposon families of IS630-Tc1-mariner (ITm), Molecular Phylogenetics and Evolution, 2023 Aug 14:107906. https://doi.org/10.1016/j.ympev.2023.107906.
- Ullah, Numan, Naisu Yang, Zhongxia Guan, Kuilin Xiang, Yali Wang, Mohamed Diaby, Cai Chen, Bo Gao, and Chengyi Song*. Comparative Analysis and Phylogenetic Insights of Cas14-Homology Proteins in Bacteria and Archaea. Genes. 2023; 14(10):1911. https://doi.org/10.3390/genes14101911.
- He, Jia, Miao Yu, Chenglin Chi, Zhanyu Du, Yao Zheng, Cai Chen, Ali Shoaib Moawad, Chengyi Song, and Xiaoyan Wang*. 2023. Insertion of 643bp Retrotransposon Upstream of PPARγ CDS Is Associated with Backfat of Large White Pigs. Animals 13, no. http://14: 2355. https://doi.org/10.3390/ani13142355
2022
- Jia, Wenzhu, Emmanuel Asare, Tao Liu, Pingjing Zhang, Yali Wang, Saisai Wang, Dan Shen, Csaba Miskey, Bo Gao, Zoltán Ivics, Qijun Qian, and Chengyi Song*. 2022. Horizontal Transfer and Evolutionary Profiles of Two Tc1/DD34E Transposons (ZB and SB) in Vertebrates. Genes 13, no. 12: 2239. https://doi.org/10.3390/genes13122239
- Chi, Chenglin, Jia He, Zhanyu Du, Yao Zheng, Enrico D’Alessandro, Cai Chen, Ali Shoaib Moawad, Emmanuel Asare, Chengyi Song, and Xiaoyan Wang*. 2022. Two Retrotransposon Elements in Intron of Porcine BMPR1B Is Associated with Phenotypic Variation. Life 12, no. 10: 1650. https://doi.org/10.3390/life12101650
- Wang, Xiaoyan, Chengling Chi, Jia He, Zhanyu Du, Yao Zheng, Enrico D’Alessandro, Cai Chen, Ali Shoaib Moawad, Emmanuel Asare, and Chengyi Song*. 2022. SINE Insertion May Act as a Repressor to Affect the Expression of Pig LEPROT and Growth Traits. Genes 13, no. 8: 1422. https://doi.org/10.3390/genes13081422.
- Du, Zhanyu, Enrico D’Alessandro, Emmanuel Asare, Yao Zheng, Mengli Wang, Cai Chen, Xiaoyan Wang, and Chengyi Song*. 2022. Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes. Genes 13, no. 8: 1359. https://doi.org/10.3390/genes13081359
- Mohamed Diaby, Zhongxia Guan, Shasha Shi, Yatong Sang, Saisai Wang, Yali Wang, Wencheng Zong, Numan Ullah, Bo Gao, and Chengyi Song*. 2022. Revisiting the Tigger Transposon Evolution Revealing Extensive Involvement in the Shaping of Mammal Genomes. Biology 11, no. 6: 921. https://doi.org/10.3390/biology11060921.
- Jia, Wenzhu, Zhongxia Guan, ShaSha Shi, Kuilin Xiang, Peihong Chen, Fen Tan, Numan Ullah, Mohamed Diaby, Mengke Guo, Chengyi Song, and Bo Gao*. 2022. The Annotation of Zebrafish Enhancer Trap Lines Generated with PB Transposon. Current Issues in Molecular Biology 44, no. 6: 2614-2621. https://doi.org/10.3390/cimb44060178.
- Zhongxia Guan, Shasha Shi, Mohamed Diaby, Patrick Danley, Numan Ullah, Mikhail Puzakov, Bo Gao, Chengyi Song*, Horizontal transfer of Buster transposons across multiple phyla and classes of animals, Molecular Phylogenetics and Evolution, 2022, 173:107506, https://doi.org/10.1016/j.ympev.2022.107506.
- Wang, Xiaoyan, Enrico D’Alessandro, Chenglin Chi, Ali S. Moawad, Wencheng Zong, Cai Chen, and Chengyi Song*. Genetic Evaluation and Population Structure of Jiangsu Native Pigs in China Revealed by SINE Insertion Polymorphisms. Animals, 2022, 12, no. 11: 1345. https://doi.org/10.3390/ani12111345.
- Zhanyu Du, Enrico D’Alessandro, Yao Zheng, Mengli Wang, Cai Chen, Xiaoyan Wang, Chengyi Song*. Retrotransposon insertion polymorphisms (RIPs) in pig coat color candidate genes. Animals, 2022, 12, no. 8: 969. https://doi.org/10.3390/ani12080969.
2021
- Shasha Shi, Mikhail Puzakov, Zhongxia Guan, Kuilin Xiang, Mohamed Diaby, Yali Wang, Saisai Wang, Chengyi Song, and Bo Gao*. 2021. Prokaryotic and Eukaryotic Horizontal Transfer of Sailor (DD82E), a New Superfamily of IS630-Tc1-Mariner DNA Transposons. Biology 10, no. 10: 1005. https://doi.org/10.3390/biology10101005
- Liu, Yibing, Wencheng Zong, Mohamed Diaby, Zheguang Lin, Saisai Wang, Bo Gao, Ting Ji, and Chengyi Song*. 2021. Diversity and Evolution of pogo and Tc1/mariner Transposons in the Apoidea Genomes. Biology 10, no. 9: 940. https://doi.org/10.3390/biology10090940
- Yali Wang, Dan Shen, Numan Ullah, Mohamed Diaby, Bo Gao, Chengyi Song*. Characterization and expression pattern of ZB and PS transposons in zebrafish. Gene Expr Patterns. 2021 Sep 1;42:119203. https://doi.org/10.1016/j.gep.2021.119203.
- Wang X, Chen Z, Murani E, D’Alessandro E, An Y, Chen C, Li K, Galeano G, Wimmers K, Song C*. A 192 bp ERV fragment insertion in the first intron of porcine TLR6 may act as an enhancer associated with the increased expressions of TLR6 and TLR1. Mobile DNA. 2021 Aug 18;12(1):20. https://doi.org/10.1186/s13100-021-00248-w.
- Naisu Yang, Bohao Zhao, Yang Chen, Enrico D’Alessandro, Cai Chen, Ting Ji, Xinsheng Wu, Chengyi Song*, Distinct Retrotransposon Evolution Profile in the Genome of Rabbit (Oryctolagus cuniculus), Genome Biology and Evolution, Volume 13, Issue 8, August 2021, evab168, https://doi.org/10.1093/gbe/evab168
- Cai Chen, Enrico D’Alessandro, Eduard Murani, Yao Zheng, Domenico Giosa, Naisu Yang, Xiaoyan Wang, Bo Gao, Kui Li, Klaus Wimmers & Chengyi Song*. SINE jumping contributes to large-scale polymorphisms in the pig genomes. Mobile DNA, 2021 Jun 28;12(1):17. https://doi.org/10.1186/s13100-021-00246-y.
- Chen, Cai, Yao Zheng, Mengli Wang, Eduard Murani, Enrico D’Alessandro, Ali S. Moawad, Xiaoyan Wang, Klaus Wimmers, and Chengyi Song* . SINE Insertion in the Intron of Pig GHR May Decrease Its Expression by Acting as a Repressor. Animals. 2021 Jun 23;11(7):1871. https://doi.org/10.3390/ani11071871.
- Chen, Cai, Xiaoyan Wang, Wencheng Zong, Enrico D’Alessandro, Domenico Giosa, Yafen Guo, Jiude Mao, and Chengyi Song* . Genetic Diversity and Population Structures in Chinese Miniature Pigs Revealed by SINE Retrotransposon Insertion Polymorphisms, a New Type of Genetic Markers. Animals. 2021 Apr 15;11(4):1136. https://doi.org/10.3390/ani11041136.
- Saisai Wang, Mohamed Diaby, Mikhail Puzakov, Numan Ullah, Yali Wang, Patrick Danley, Cai Chen, Xiaoyan Wang, Bo Gao, Chengyi Song*, Divergent evolution profiles of DD37D and DD39D families of Tc1/mariner transposons in eukaryotes, Molecular Phylogenetics and Evolution, 2021 Mar 10:107143. https://doi.org/10.1016/j.ympev.2021.107143.
- Dan Shen, Chengyi Song, Csaba Miskey, Shuheng Chan, Zhongxia Guan, Yatong Sang, Yali Wang, Cai Chen, Xiaoyan Wang, Ferenc Müller, Zoltán Ivics, Bo Gao* , A native, highly active Tc1/mariner transposon from zebrafish (ZB) offers an efficient genetic manipulation tool for vertebrates, Nucleic Acids Research, 2021 Feb 26;49(4):2126-2140. https://doi.org/10.1093/nar/gkab045.
2020
- Bo Gao; Wencheng Zong; Csaba Miskey; Numan Ullah; Mohamed Diaby; Cai Chen; Xiaoyan Wang; Zoltán Ivics; Chengyi Song*. Intruder (DD38E), a recently evolved sibling family of DD34E/Tc1 transposons in animals. Mobile DNA, 2020 Dec 10;11(1):32. https://doi.org/10.1186/s13100-020-00227-7
- Bo Gao, Yali Wang, Mohamed Diaby, Wenchen Zong, Dan Shen, Saisai Wang, Cai Chen, Xiaoyan Wang, Chengyi Song*. Evolution of pogo, a separate superfamily of IS630-Tc1-mariner transposons, revealing recurrent domestication events in vertebrates. Mobile DNA, 2020 Jul 22;11:25. https://doi.org/10.1186/s13100-020-00220-0
- Bo Gao, Yatong Sang, Wencheng Zong, Mohamed Diaby, Dan Shen, Saisai Wang, Yali Wang, Cai Chen, Chengyi Song*. Evolution and Domestication of Tc1/mariner transposons in the African coelacanth (Latimeria chalumnae) genome. Genome, 2020 Aug;63(8):375-386. https://doi.org/10.1139/gen-2019-0216.
- Dan Shen, Bo Gao, Csaba Miskey, Cai Chen, Yatong Sang, Wencheng Zong, Saisai Wang, Yali Wang, Xiaoyan Wang, Zoltán Ivics, Chengyi Song*, Multiple Invasions of Visitor, a DD41D Family of Tc1/mariner Transposons, throughout the Evolution of Vertebrates, Genome Biology and Evolution, 2020 Jul 1;12(7):1060-1073. https://doi.org/10.1093/gbe/evaa135
- Wencheng Zong, Bo Gao, Mohamed Diaby, Dan Shen, Saisai Wang, Yali Wang, Yatong Sang, Cai Chen, Xiaoyan Wang, Chengyi Song*. Traveler, a New DD35E Family of Tc1/Mariner Transposons, Invaded Vertebrates Very Recently. Genome Biology and Evolution, 2020 Mar 1;12(3):66-76. https://doi.org/10.1093/gbe/evaa034.
2019 and before
- Yatong Sang, Bo Gao, Mohamed Diaby, Wencheng Zong, Cai Chen, Dan Shen, Saisai Wang, Yali Wang, Zoltán Ivics & Chengyi Song*. Incomer, a DD36E family of Tc1/mariner transposons newly discovered in animals. Mobile DNA, 2019 Nov 23;10:45. https://doi.org/10.1186/s13100-019-0188-x.
- S. Wang, Y. Wang, D. Shen, L. Zhang, W. Chen, S. Chan, Z. Guan, C. Song & B. Gao*. ZB transposon and chicken vasa homologue (Cvh) promoter interact to increase transfection efficiency of primordial germ cells in vivo, British Poultry Science, 2019 Dec;60(6):724-728. https://doi.org/10.1080/00071668.2019.1639138.
- Cai Chen, Wei Wang, Xiaoyan Wang, Dan Shen, Saisai Wang, Yali Wang, Bo Gao, Klaus Wimmers, Jiude Mao, Kui Li & Chengyi Song*. Retrotransposons evolution and impact on lncRNA and protein coding genes in pigs. Mobile DNA. 2019 May 6;10:19. https://doi.org/10.1186/s13100-019-0161-8.
- Dan Shen, Songlei Xue, Shuheng Chan, Yatong Sang, Saisai Wang, Yali Wang, Cai Chen, Bo Gao, Ferenc Mueller, Chengyi Song*. Enhancer Trapping and Annotation in Zebrafish Mediated with Sleeping Beauty, piggyBac and Tol2 Transposons. Genes. 2018 Dec 13;9(12):630. https://doi.org/10.3390/genes9120630.
- Bo Gao, Saisai Wang, Yali Wang, Dan Shen, Songlei Xue, Cai Chen, Hengmi Cui & Chengyi Song*. Low diversity, activity, and density of transposable elements in five avian genomes. Functional & Integrative Genomics. 2017 Jul;17(4):427-439. https://doi.org/10.1007/s10142-017-0545-0.
- Bo Gao; Dan Shen; Songlei Xue; Cai Chen; Hengmi Cui; Chengyi Song*. The contribution of transposable elements to size variations between four teleost genomes, Mobile DNA. 2016 Feb 9;7:4. https://doi.org/10.1186/s13100-016-0059-7.
- 桑亚通,沈丹,陈伟,产舒恒,顾浩,高波,宋成义*,Tol2转座子介导斑马鱼rps26基因附近增强子捕获及注解分析,生物工程学报,2018,34(3):449-458
- 沈丹,陈才,王赛赛,陈伟,高波,宋成义*,Tc1_Mariner转座子超家族的研究进展,遗传,2017, 39(1):1-13
- 谢飞,高波,宋成义*,陈国宏,“睡美人”转座子的研究进展,遗传,2007,29(7):785-792