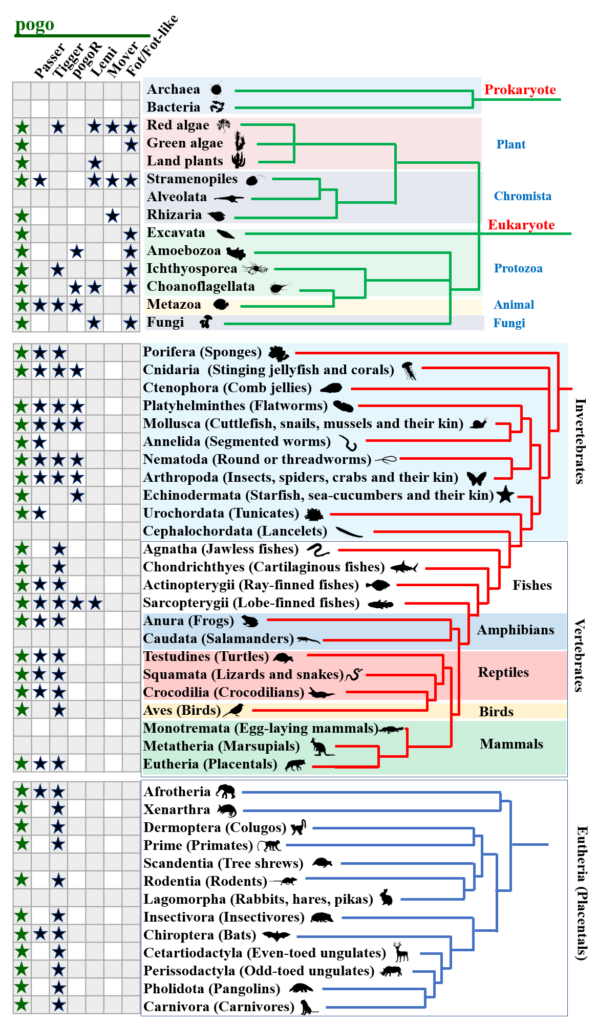
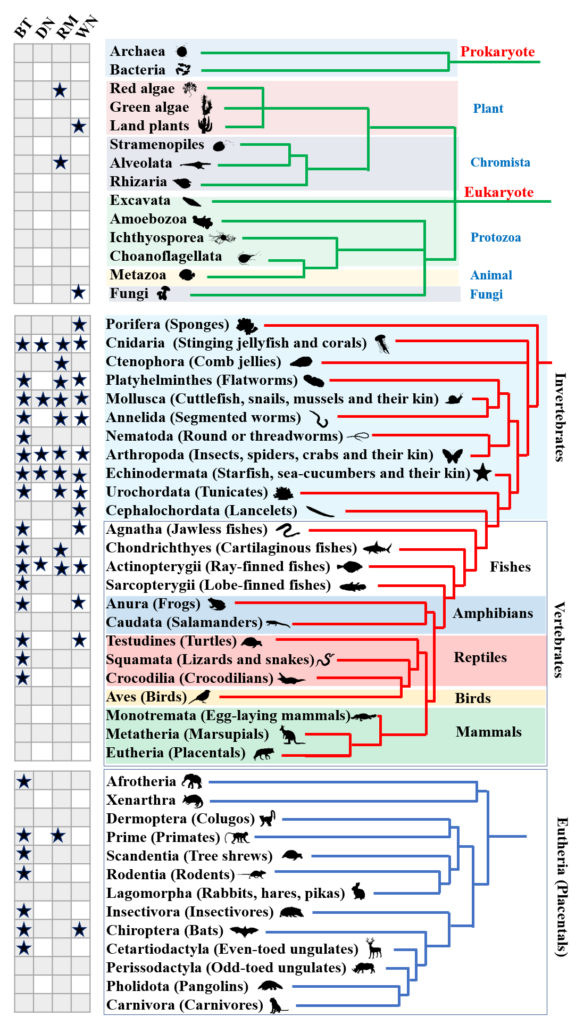
一、博士及博士后研究方向
研究方向1:靶向DNA转座子挖掘及高效安全载体递送技术研发
主要开展靶向DNA转座子挖掘、活性转座子验证筛选、系统优化和转座酶定向进化,为人类基因治疗和生物转基因研究提一种安全高效的靶向非病毒载体递送系统,为实现较大基因片段在细胞基因组中的高效定点整合,破解制约众多基因治疗和基因组改造应用的关键瓶颈,释放其在生物转基因育种和基因治疗等应用方向巨大潜力提供技术积累。
研究方向2:转座子起源小分子靶向核酸酶挖掘及基因编辑工具开发
通过对原核生物基因组大数据挖掘,揭示IS605,IS607和IS1341转座子起源小分子核酸酶(TnpB和IscB)的系统进化和结构特征,筛选具有开发潜力的小分子核酸酶进行定向进化、分子重构,研发基因编辑效率高、靶向特异性强的新型基因编辑工具,为人类基因治疗和转基因生物育种提供更加安全高效的编辑系统。
研究方向3:基于猪基因组中活性逆转座子插入多态(RIP)为基础的分子标记研发、RIP液相芯片及育种应用评估
主要开展猪等家畜基因组转座子注释,逆转座子介导产生分子标记(也称为转座子插入多态RIP,Retrotransposon insertion polymorphism)规模挖掘及其遗传效应研究,RIP标记配套液相芯片研发及其在育种中的应用评估。
二、博士申请(硕博连读和申请考核)要求
参照学校和学院每年发布的当年申请要求,一般应达到以下要求:
硕博连读基本条件
(一)拥护中国共产党的领导,具有正确的政治方向,热爱祖国,愿意为社会主义现代化建设服务,遵纪守法,品行端正。无任何考试作弊、学术剽窃及其它违法违纪行为。
(二)身体和心理健康状况符合我校体检要求。按照教育部、卫生部、中国残联印发的《普通高等学校招生体检工作指导意见》(教学〔2003〕3号)、《教育部办公厅卫生部办公厅关于普通高等学校招生学生入学身体检查取消乙肝项目检测有关问题的通知》(教学厅〔2010〕2号)规定执行。
(三)英语水平。通过CET-4或具有一年及以上的国外留学经历。
(四)学分课(学术型、专业型分别排名)成绩排名年级前50%,且没有考试不及格的课程。
(五)在满足上述条件的前提下,英语通过CET-6,或近三年雅思(IELTS)成绩≥6.5分或托福(TOEFL)成绩≥85或GRE成绩≥260;或以第一作者发表SCI论文(需Online,截止日期12月31日);或授权发明专利(排名前2名,截止日期12月31日)者,优先考虑。
申请考核基本要求
专业考核包括申请者科研成果、英语水平审核和面试考核。
(一)科研成果
1.以第一作者身份发表SCI收录论文1篇,或CSCD(核心库)收录论文1篇,或北大中文核心收录论文2篇;
2.在满足条件1的前提下,有授权发明专利(排名前2名)者优先。
(二)英语水平
英语水平达到以下条件之一:
1.提供以下至少一项英语考试的成绩证明,具体包括:国家CET-6成绩≥426分,或近三年雅思(IELTS)成绩≥6.5分或托福(TOEFL)成绩≥85(老TOEFL成绩≥560分);
2.在英语国家或地区获得硕士学位;
3.具有一年及以上的英语国家国外留学经历;
4.发表SCI三区(中科院分区)以上(含三区)论文1篇,且国家CET-4成绩≥426分。
三、博士后招聘要求
1、具有博士学位,品学兼优,身体健康,年龄不超过35周岁。
2、获得博士学位年限不超过两年。
3、全职全时,不招收在职博士后。
4、专业背景(生物学、遗传学、发育生物学、细胞生物学、生物化学与分子生物学、生物物理学、生物信息学、动物遗传育种),熟悉哺乳动物细胞培养或者生物信息学分析优先考虑。
5、学术要求(以下两项需满足一项即可):
(1)申请师资博士后需以第一作者在SCI期刊上发表论文至少2篇,且Top期刊研究论文1篇,或一区研究论文1篇。
(2)申请全职博士后需以第一作者在SCI期刊上发表研究论文2篇或二区以上研究论文1篇。
6、符合扬州大学博士后管理的规定
四、博士后应聘材料
1、个人简历,包括个人基本情况、学习工作经历、主要研究工作内容、博士在学期间发表的第一作者论著清单(SCI收录文章需注明影响因子)、获得的奖励情况。
2、博士学位证书、毕业证书(应届毕业生可于毕业后补发)。
3、博士论文全文PDF版(应届毕业生可于毕业后补发)。
4、博士在学期间发表的第一作者SCI收录论著全文。
5、两封专家推荐信(包括博士导师的推荐信)。
6、博士后工作期间研究设想。
五、待遇
1、博士的奖助学金参照学校和学院说明。
2、博士后按照扬州大学相关福利待遇规定执行,税前基本年薪25-30万元/年,详见学校博士后工作 (yzu.edu.cn)。
2、在享受学校相关待遇基础上,课题组将另提供与其业绩相称的年终绩效奖励。
六、应聘方式
1、招聘长期有效,有意向者,请将详细的个人简历(包括完整的学习工作经历及代表成果清单)以邮件形式发送,邮件主题注明“申请扬州大学转座子实验室博士后/博士”;
2、联系人:宋老师 Email:cysong@yzu.edu.cn
国外学术岗位招聘
Job in Bioloxy
https://professorpositions.com/n1388548